


Vitreous abnormalities are common with a high risk of retinal detachment. Some individuals exhibit congenital abnormalities of the anterior chamber drainage angle, increasing the risk for glaucoma. Congenital cataracts may also be present. High myopia is typically congenital and may be associated with astigmatism. Individuals with SS are commonly myopic (>−3.00 diopters) and are at high risk for ocular complications, including vitreous abnormalities, retinal detachment, glaucoma, and cataracts. A family history is often absent in recessive cases, and clinical phenotypes may be less distinctive than those with STL1 and STL2. Biallelic variants in candidate genes, including LOX元, a lysly oxidase gene important for proper cross-linking of type II collagen, and LRP2, an endoplasmic transmembrane receptor gene associated with facio-oculo-acoustico-renal (FOAR) syndrome and Donnai–Barrow Syndrome (DBS), have also been reported in individuals with SS-like phenotypes. Biallelic variant in COL11A1 have also been reported in cases of AR SS, although typically are associated with a more severe skeletal dysplasia.
More rare than the AD forms, biallelic pathogenic variants in COL9A1, COL9A2, and COL9A3 have been reported to cause recessive forms of Stickler Syndrome (Stickler Syndromes 4-6 ). This is because, while COL11A2 is expressed in the joints, inner ear, and craniofacial structures, it is not expressed in the eye. This is an important distinction for ophthalmologists, as individuals with OSMEDA will manifest all of the findings of typical SS except for the ocular complications. A smaller proportion of AD cases are caused by heterozygous pathogenic variants in COL11A1 (Stickler Syndrome Type II ), and COL11A2 (Otospondylomegaepiphseal dysplasia, AD, formerly known as Non-Ocular Stickler Syndrome). Thus, a diagnosis of SS should be considered in an individual with suggestive ocular findings even if no systemic features are present. Ocular-only phenotypes may also be caused by variants in COL2A1 outside of exon 2. While most individuals with pathogenic COL2A1 variants will exhibit systemic manifestations of SS, individuals harboring pathogenic variants in exon 2 will exhibit an ocular-only phenotype due to alternative splicing of the gene. STL1 is the classic AD type, caused by heterozygous pathogenic variants in COL2A1. Stickler Syndrome type I (STL1) is the most common form of SS, accounting for approximately 80–90% of cases. A high index of suspicion allows for early detection and diagnosis which can improve clinical outcomes for these individuals. However, penetrance does appear to be complete, meaning all affected individuals will manifest some features of the condition. SS exhibits significant inter- and intrafamilial phenotypic variability, meaning that individuals with the same pathogenic variant may exhibit different clinical manifestations, even within families. These genes are associated with the formation of collagens type II, IX, and XI, which are expressed within the vitreous, skeleton, and inner ear, with limited genotype-phenotype correlation.

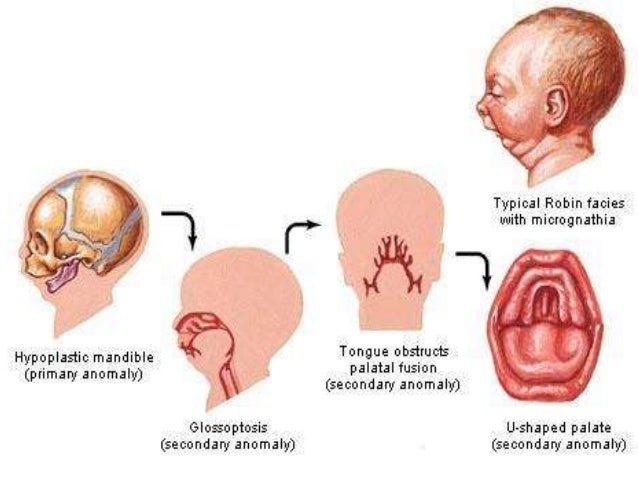
Furthermore, SS exhibits genetic heterogeneity, as to date, pathogenic variants in 6 different genes can cause SS. While the most common forms are autosomal dominant (AD), other forms are inherited in an autosomal recessive (AR) manner. Therefore, it should not be surprising that SS may present with a wide range of findings, including micrognathia (alone or as part of Pierre Robin sequence), cleft palate, hearing loss, or early onset osteoarthritis, either in the proband or an affected family member. While ocular complications are both common and often very serious, skeletal/joint, inner ear, and craniofacial structures are often involved. First described in 1965 by Gunnar Stickler, SS is best known to ophthalmologists as a condition that confers a risk for significant ocular complications, ranging from severe myopia to retinal detachment and vision loss. Stickler Syndrome (SS) is a relatively common multisystem connective tissue disorder.


 0 kommentar(er)
0 kommentar(er)
